Pooled platelet psoralen-treated (PPPT) manufacturing
The manufacturing of PPPT begins with the collection of whole blood into buffy coat collection sets from donors in a Canadian Blood Services donor centre. Whole blood units are centrifuged to separate the plasma, buffy coat (containing leukocytes and platelets) and red blood cell fractions. The red cell and plasma layers are extracted out of the collection bag leaving behind the buffy coat layer with a small amount of plasma and red blood cells (also referred to as the B1 method as described for whole blood buffy coat collections). Seven buffy coats—one from each donor unit— are then pooled together and platelet additive solution (PAS-E) is added. The buffy coat pool is then centrifuged, and the platelet-rich supernatant (comprised of residual plasma and PAS-E) is extracted from the red blood cells retained in the buffy coat through a platelet-sparing leukoreduction filter, to produce a double dose pooled platelet.
Amotosalen is then added to the double dose pooled platelet unit, and the double dose pooled platelet unit undergoes treatment with UVA illumination to facilitate crosslinking of amotosalen to residual nucleic acid material within the unit. A single treatment with UVA can adequately intercalate DNA and RNA from donor cells and pathogens within a range of concentrations. Residual amotosalen and its photoproducts are then removed by incubating the double dose pooled platelet with a compound adsorption device. The double-dose PPPT unit is then split into two single-dose PPPT units.
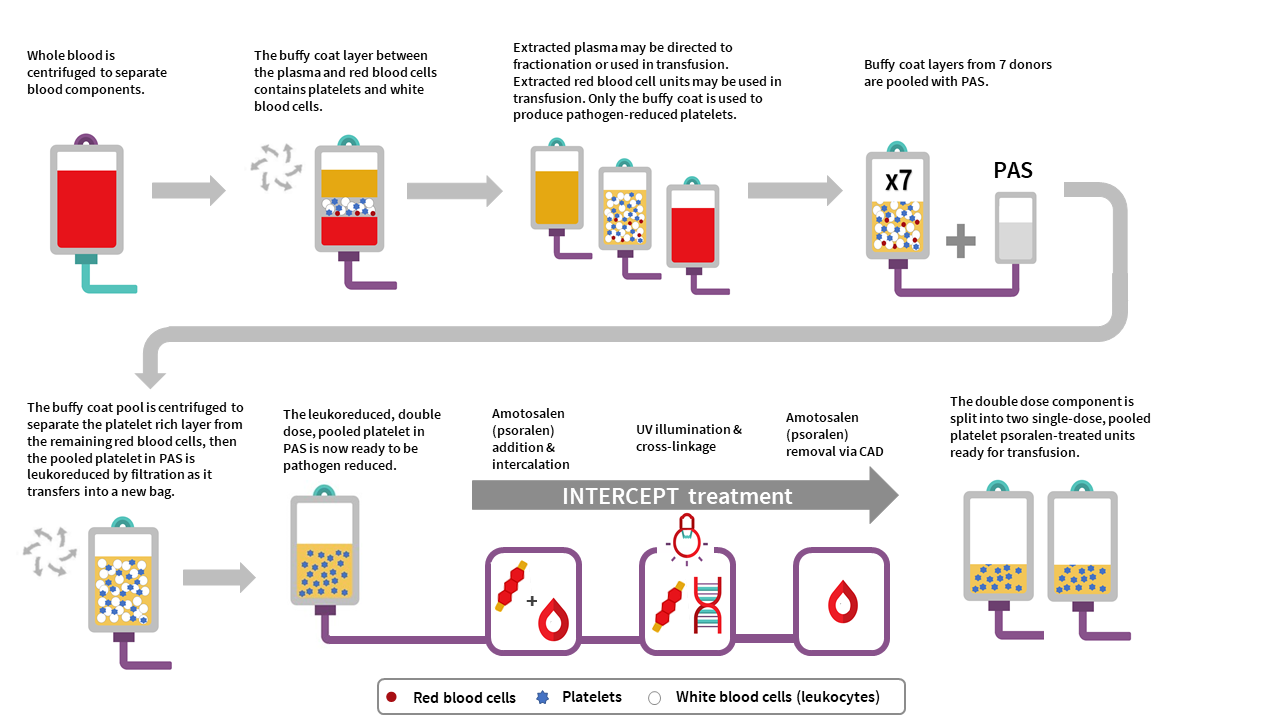
Figure 1: Pooled platelets psoralen-treated (PPPT) manufacturing at Canadian Blood Services
Additional information can be found in the clinical guide to transfusion.