Stealth blood cells: Fooling the immune system to make transfusion safer for hard-to-match patients
Wednesday, April 27, 2016 Michelle Hampson
A Q&A with Dr. Mark Scott follows.
By Michelle Hampson
When a person is in dire need of blood, a blood transfusion seems like a simple solution. A donor donates blood, and eventually a patient in need receives it. Yet, in reality this life-saving medical procedure, as safe as it may be, is not that simple.
Blood cells are complex
Each person’s red blood cells are coated in proteins and carbohydrates called antigens. The types of antigens can vary greatly from person to person. Our immune system recognizes the antigens on our own blood cells as “self”, but may not recognize all of those on a donor’s red blood cells, identifying it as “foreign”. If the immune system detects “foreign” antigens, it launches an attack, a medical emergency called alloimmunization that can leave blood recipients very sick.
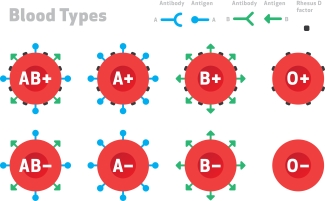
Many people know about the ABO blood groups (blood types A, B, AB and O). But in reality, that’s just a really small subset of the groups of antigens that we have on our red blood cells. There are about 35 different red blood cell group families and over 300 different unique antigens. And so when we transfuse individuals, we usually do it based on ABO or Rh (positive or negative). But occasionally, especially in people who have received a lot of transfusions, their immune system will recognize some of these non-ABO antigens as foreign and will make antibodies to it.
“There are people in the world that have such unique blood cells in terms of their antigens, or lack of antigens, that we cannot find compatible blood for them. These individuals, if they become sick or injured, they are at a much greater risk of dying simply because blood banks don’t have blood to give to them,” explains Dr. Scott. “And this is where stealth blood cells can come into play.”
What if a donor’s blood cells could hide from the recipient’s immune system?
Researchers have developed a technique that could make blood transfusions around the world much safer and more accessible, regardless of a donor or recipient’s blood type. By “chemically gluing” one simple compound to the blood cells, researchers can create stealth red bloods cells that can trick the immune system. These cells can easily hide from the immune system, undetected.
The compound is called methoxypolyethylene glycol (mPEG). It is very common, found in food and a variety of medicines, and therefore most peoples’ immune systems tolerate it. When an mPEG molecule is “glued” to a red blood cell, it works in two ways to escape immune cells that may seek to destroy it.
While most other molecules that could sit on the surface of a cell are rigid, mPEG is very flexible. This flexibility allows the molecule to flail randomly in every direction, creating a physical barrier between the red blood cell and any immune cells that approach it.
As well, many immune cells detect foreign cells based on the positive and negative charges of its antigens. Immune cells use these different charges and their distribution to identify each cell (similar to how fingerprints are used to identify individual people), as well as to bind to the cell. But mPEG is neutrally charged, allowing red blood cells with the molecule to evade detection from immune cells.
Pioneering the discovery of stealth red cells
Dr. Mark Scott is a senior scientist with Canadian Blood Services and an investigator at the Centre for Blood Research at the University of British Columbia. He is also a pioneer of stealth red blood cells. He first came across PEGylated compounds many years ago. Upon noticing how gluing mPEG to an enzyme helped it evade the immune system, he wondered if the same thing could happen with PEGylated red blood cells.
In his first study exploring PEGylated red blood cells, Dr. Scott and his colleagues found that adding the compound to red blood cells did not affect their structure, and their ability to carry oxygen remained within the normal range. They injected PEGylated red blood cells from humans into mice, and the mice did not experience an immune response to the foreign blood.
If stealth red blood cells offer similar benefits in humans, the implications could be huge for thousands of people around the world.
“There are people in the world that have such unique blood cells in terms of their antigens, or lack of antigens, that we cannot find compatible blood for them. These individuals, if they become sick or injured, they are at a much greater risk of dying simply because blood banks don’t have blood to give to them,” explains Dr. Scott. “And this is where stealth blood cells can come into play.”
People with rare blood types and unique antigens are not the only ones who could benefit from this technology. A number of diseases, such as thalassemia or sickle cell anemia, require patients to undergo blood transfusions quite frequently, sometimes as often as 30 times per year.

Dr. Scott notes that these patients who receive chronic transfusions are the most likely to benefit from stealth red blood cells. “Because they receive so much blood, their immune system has a much higher exposure the foreign blood antigens, and is much more likely to somewhere along the line suddenly go – hey, you’re not normal, I don’t like you, and I’m going to make an antibody against you.”
For example, a study in 2013 found that 58 per cent of sickle cell anemia patients who received chronic blood transfusions became alloimmunized, which makes subsequent transfusions problematic.
Next steps in fooling the immune system…
Now Dr. Scott and his team are collecting antibodies from a diverse group of people in the hopes of mapping out how effective PEGylated red blood cells are for different blood groups.
Since studying mPEG as an effective compound for camouflaging red blood cells, Dr. Scott and his team have expanded their work to camouflage white blood cells, those of the immune system.
The results are remarkable. Normally, when you put white blood cells from two different people in a test tube together, the cells will proliferate like crazy, generating more immune cells in order to wipe out the other “foreign” cells. The immune reaction turns into a type of warfare. But in a study by Dr. Scott and his colleagues, when one “side” of white blood cells was PEGylated this arms race and attack on one another did not occur. Videos at the microscopic level reveal that PEGylation decreases the number of interactions between the two opposing white blood cells.
“In one of the movies where we have one of the populations PEGylated, you see that the white blood cells don’t interact with each other. So we’re camouflaging the cells to the point that neither population realizes the other population is different and is there,” Dr. Scott says.
What’s more, adding a third strain of white blood cells in live mice, even though this strain was not PEGylated, did not cause an immune response. It seems that the presence of the PEGylated white blood cells allowed the immune system of the mouse to become accustomed to foreign blood cells. “So what we’ve done is we’ve induced tolerance in the blood recipient,” Dr. Scott explains.
Modifying white blood cells could not only help people who need blood transfusions, but people who need other tissue transplants as well. (Blood transfusion is technically a type of tissue transplant).
Although more research is needed before stealth red blood cells are tested in humans, the initial data suggest an intriguing way to avoid the negative reactions that can occur with blood transfusions and make the process much simpler – no matter how complex a person’s blood may be.
Further reading: Scott MD: ResearchUnit: Using immunocamouflage to fool the immune system. 2015.

Q & A with Dr. Mark Scott
Why must patients receive certain types of blood?
Many people know about the ABO blood groups (blood types A, B, AB and O). But in reality, that’s just a really small subset of the groups of antigens that we have on our red blood cells. There’s about 35 different red blood cell group families and over 300 different unique antigens. And so when we transfuse individuals, we usually do it based on ABO or Rh (positive or negative). But occasionally, especially in people who have received a lot of transfusions, their immune system will recognize some of these non-ABO antigens as foreign and will make antibodies to it. And when they make antibodies to it, these antibodies bind to the donor blood and result in the clearance of the donor blood and could make the patient very sick.
How did you first start exploring immunocamouflage of red blood cells?
Way back in the dark ages, when I was a graduate student at the University of Minnesota, I had done some work as part of my PhD thesis on an enzyme called superoxide dismutase (SOD). But I had gotten some SOD that was PEGlyated. It’s when they take a molecule called methoxypolyethylene glycol (mPEG), and covalently glued it to the SOD molecule. And this made the SOD molecule invisible to the immune system… So even though that was probably 20 years or so in the past, I thought about that when I was at Albany Medical College in New York. That’s where we started our work on immunocamouflage. We discovered that when we glued this mPEG to a red blood cell, we basically camouflaged all of these groups of antigens. And when we camouflaged the blood group antigens, we could prevent immune recognition of these red blood cells in a living animal. In our case we used mice.
So how does immunocamouflage of red blood cells work exactly?
Well, the mPEG is a special kind of molecule. A lot of polymers are single chains and they have a rigid shape to them. So think of spaghetti before you cook it…. But the mPEG molecule is like that cooked piece of spaghetti, it’s very flexible. So when we attached the mPEG molecule to the red blood cell, that little strand of polymer is whipping around in its environment and that creates a space-filling capacity. It’s basically beating back anything that’s trying to approach the cell surface by its random motion.
mPEG is also good at camouflaging the charge of the cell. So every cell has a bunch of (positive and negative) charges scattered around in various locations and these charges are very important in terms of the immune system recognizing things as either normal or foreign. The mPEG polymer is neutrally charged, so it camouflages what the charges of the cell surface are. In essence it’s like a magnet on a refrigerator. The magnet wants to stick but if you put a fairly think piece of plastic between the magnet and the refrigerator, the magnet can’t sense the charge of the metal door and it can’t stick.
What do you find most fascinating about your work?
Blood transfusion is a tissue transplant, and it’s and immunologically complex tissue. It’s amazing that we’ve had the success that we have had with blood transfusions over time. It has been enjoyable from a science perspective, and from the perspective of knowing that this could transition into changes in clinical care and improving peoples’ lives.
What are your next steps with this research?
In terms of red blood cells, we continue to gather alloantibodies from individuals because we would like to map out how effective the camouflaging technology is against different blood group families and antibodies to those blood group families.
Dr. Mark Scott has pioneered the immunocamouflage of red blood cells, leukocytes and platelets. This novel, patented technology relies on the chemical attachment of biologically safe polymers on cell membranes to camouflage cell surface antigens. He is a senior scientist with Canadian Blood Services and an investigator at the Centre for Blood Research at the University of British Columbia.
Canadian Blood Services – Driving world-class innovation
Through discovery, development and applied research, Canadian Blood Services drives world-class innovation in blood transfusion, cellular therapy and transplantation—bringing clarity and insight to an increasingly complex healthcare future. Our dedicated research team and extended network of partners engage in exploratory and applied research to create new knowledge, inform and enhance best practices, contribute to the development of new services and technologies, and build capacity through training and collaboration.
About the author
Michelle Hampson is a writer who has spent years communicating medical science and treatment options to people living with chronic conditions, such as cancer and autoimmune diseases. Now based in Washington, DC, she reports on research published in the journal Science.
The opinions reflected in this post are those of the author and do not necessarily reflect the opinions of Canadian Blood Services.
Related blog posts
Wonder drug it may be, but IVIg is a slippery fish. Even after 60 years, little is known about precisely how it works. An encounter with a scientist The first thing you notice when you walk into Dr. Don Branch’s office at 67 College Street in Toronto is how small it seems. And colourful, owing to an...
Images from the Lazarus Research Group lab show some fascinating and potentially life-saving science in action.