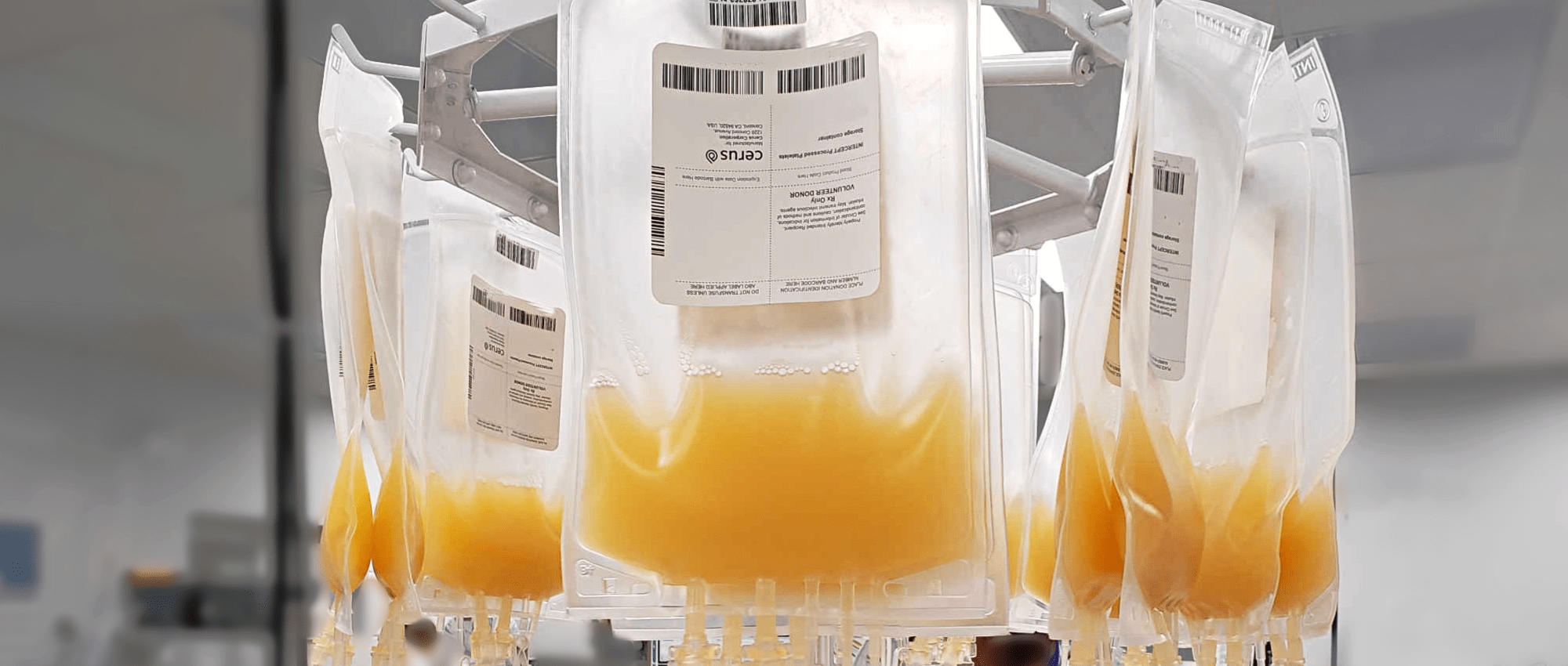
Introducing pathogen-reduced platelets to Canada
Providing a new layer of safety through pathogen inactivation
Canadian Blood Services works hard to protect Canada’s blood supply for the patients who rely on it. We ask all donors a series of eligibility questions, and we test all donations for an array of diseases. We have a team that watches patterns of diseases and works hard to identify emerging threats. But new diseases arise, and there are some diseases for which we can’t test.
What if we could zap any viruses, bacteria or parasites that somehow make it through the existing layers of safety? The technology exists for platelets and plasma, and is in development for red cells, too.
This week, Canadian Blood Services launched its first pathogen-reduced fresh blood product. Pathogen-reduced platelets are now on the shelves of hospitals served by our Ottawa production site. We are assessing plans for further expansion of this process to all Canadian Blood Services production sites.
“The safety of the blood supply is paramount to Canadian Blood Services. Pathogen inactivation adds a new layer of safety, especially against pathogens that are new or emerging, as well as those for which there is no available test,” says Dr. Chantale Pambrun, senior medical director of innovation and portfolio management.
What is pathogen inactivation?
Canadian Blood Services intends to introduce pathogen-reduced solutions for all our fresh blood components — platelets, plasma and red cells — as Health Canada-licensed solutions become available.
The pathogen inactivation technology Canadian Blood Services is using targets and damages the genetic material in pathogens such as viruses, bacteria and parasites using ultraviolet (UV) light. This stops the pathogen from being able to cause illness.
This technology is new to Canada but has been used routinely in Europe for well over a decade, and is increasingly being used in the U.S.

Product process development specialist Stacey Hayes (left) and lab assistant Kim Manderson (right) place a unit of pooled platelets into the illuminator for its pathogen inactivation treatment.
Why start with platelets?
Platelets are the only fresh blood product that must be stored at room temperature rather than in a fridge or freezer. Because of this, they are at a higher risk of bacteria growth and therefore a higher risk of an adverse transfusion event in patients.
Platelets are the component of blood that helps with clotting. If an injury or blood loss occurs, platelets are activated, and a person’s blood begins to clot to prevent excessive bleeding.
In a healthy person, a large number of platelets circulate in the body. Patients who have low platelet counts (e.g. cancer patients undergoing chemotherapy or following excessive bleeding) or platelets that don't function properly need platelet transfusions as part of their treatment. Canadian Blood Services manufactures approximately 115,000 platelet units every year.
Currently, there are no available pathogen inactivation technologies for red cells in Canada. Existing pathogen inactivation technologies use UV light. Plasma is a liquid and light can shine through it easily. Platelets are very small, and each platelet bag is also relatively small, so light passes through it as well. Red cells, however, are large and densely packed. This makes it hard to destroy all potential pathogens consistently.
Work to develop pathogen inactivation technology for red cells is well underway by the industry, and we will continue to work to ensure those developing the technology are looking at the Canadian market and introducing a Health Canada-approved solution for the benefit of patients.