This post was written with support from Dr. Denise Landry, Senior Research Assistant, Canadian Blood Services.
The International Collaboration for Transfusion Medicine Guidelines (ICTMG) recently convened a panel to provide guidance for fetal neonatal alloimmune thrombocytopenia (FNAIT). Findings from a systematic review have been recently published in the journal Blood.
What is FNAIT?
Fetal neonatal alloimmune thrombocytopenia – aka FNAIT – is a condition that can cause a severe bleeding disorder in fetuses and newborns. In FNAIT, the mother’s antibodies cross the placenta and bind to the baby’s platelets, making them appear “foreign”. The baby’s platelets are then targeted by the immune system and cleared from the baby’s circulation. This can lead to the baby having a low platelet count (thrombocytopenia), bruising, a rash called petechiae or bleeding, and possibly bleeding into the brain.
Who is at risk?
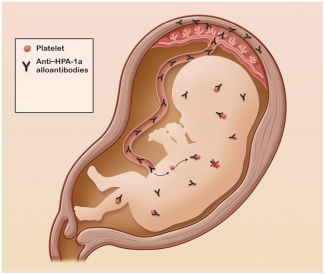
FNAIT affects about 1 in 2000 live births and can be life-threatening. Intracranial (within the skull) bleeds are a major risk and occur in about 10 to 20 per cent of FNAIT cases. These can lead to brain damage and, in rare cases, death of the fetus.
Treatment options
Intravenous Immunoglobulin (IVIG) – a solution of concentrated antibodies that come from donated plasma – is one of the main treatment options for mothers with FNAIT. Other options are fetal blood sampling with intrauterine platelet transfusions.
Which treatment is best?
The International Collaboration for Transfusion Medicine Guidelines (ICTMG), a group of specialists comprising hematologists, hematopathologists, obstetricians, pediatricians and methodologists from a variety of countries convened a panel to provide guidance for FNAIT. The goal of the ICTMG is to create and promote guidelines that optimize transfusion care around the globe.
The FNAIT Guideline Development Group recently conducted a systematic review to evaluate the various treatments for mothers with FNAIT, including IVIG, prednisone and intrauterine platelet transfusion. This work was published in the journal Blood in March 2017.
The team systematically searched the medical literature from 1946 to 2015, found 5940 citations and included 26 studies on the management of FNAIT during pregnancy. In a review of four randomized controlled trials and 22 non-randomized studies, the group discovered that fetal blood sampling, intrauterine platelet transfusions and IVIG had comparable outcomes in successfully treating FNAIT. They also discovered that fetal blood sampling and intrauterine platelet transfusions were more likely than weekly transfusions of IVIG to cause complications (11 per cent), such as preterm emergency cesarean sections because of fetal distress.
Given these findings, the team concluded that the first line of defense for pregnant mothers with FNAIT should be weekly transfusions of IVIG.
Transfusion News: Current Evidence Suggests that Non-invasive Procedures are Best to Treat Fetal and Neonatal Alloimmune Thrombocytopenia
Who will benefit from this information?
This review equips doctors with evidence-based information that will help mothers and their babies benefit from non-invasive treatment strategies like IVIG.
What further work needs to be done?
The FNAIT Guideline Development Group is also working on systematic reviews for postnatal interventions for FNAIT and for the association of maternal platelet alloantibody levels with fetal and neonatal morbidity and mortality. Once complete, recommendations from all three systematic reviews will be consolidated into a guideline document that will also highlight the several unanswered questions in this area that will need further investigation and research.
Note: A podcast and video related to FNAIT guidelines is currently in production.
Further reading
- Platelets Unplugged: The Sticky Truth – An ICTMG/AABB podcast
- Platelets vs. blood vessels: what causes bleeding in fetuses and newborns with FNAIT
About the ICTMG
Established in 2011, the ICTMG (International Collaboration for Transfusion Medicine Guidelines) is a group of specialists comprising hematologists, physicians and methodologists from a variety of countries who work together to establish evidence-based transfusion medicine guidelines. Their goal is to create and promote these guidelines to optimize transfusion care around the globe.
Canadian Blood Services is proud to support the activities of the ICTMG. Through the Centre for Innovation, we provide administrative support to this international group that includes a healthy contingent of Canadian experts.
The opinions reflected in this post are those of the author and do not necessarily reflect the opinions of Canadian Blood Services nor do they reflect the views of Health Canada or any other funding agency.
Related blog posts
The International Collaboration for Transfusion Medicine Guidelines aims to optimize transfusion care around the globe. Platelets Unplugged, co-produced with the AABB, is the first in a series of podcasts digging into best practice for the transfusion medicine community and health-care professionals.
